Empirical Formula of Benzene
Mass of molecular formula of benzene 6126168. A chemical formula showing the ratio of elements in a compound rather than the total number of atoms.
Quantitative Chemistry Molecular Formulas
It represent the actual number of atoms of all the elements present in a compound.

. Know the definition of Empirical Formula and solve examples at Embibe. To write the empirical molecular and structural formula for Benzene C6H6 well start with the molecular formulaThe molecular formula shows us the number. Empirical formula of benzene.
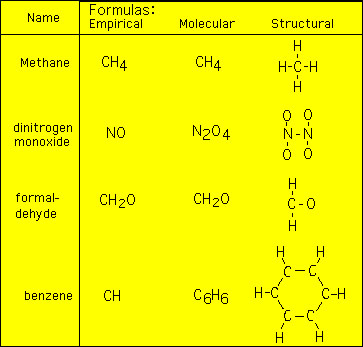
Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms. What is the chemical formula. Since the empirical formula is the lowest whole number formula the empirical formula for C6H6 would be CH divide both C and H by 6.
Thus the molar mass of benzene will be 6left 12 right 6left 1 right 78 The empirical formula is the simplest ratio of. It has a cyclic. SUBMIT ANDWER 16 Periodic Table part 1 of 3 An oxide of phosphorous.
Benzene C6H6 CID 241 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. What are compounds containing a benzene ring called. What is the ratio of empirical formula to molecular formula of benzene.
Molecular formula of the benzene is C6H6 Emperical formula. The empirical formula of benzene is CH its chemical formula is C 6 H 6. If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will be produced.
C6H6 is the molecular formula for benzene. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms. By signing up youll get thousands of step-by-step solutions to your homework.
What is the mass of 1 mole of the empirical formula of benzene. Answer in units of g. If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will be.
Arenes or aromatic compounds. The ratio of empirical formula is. What is the ratio of empirical formula to molecular formula of benzene.
What is the empirical formula of benzene C_6H_6. The empirical formula is the simplest whole number ratio defining constituent atoms in a species and thus for benzene whose molecular formula is C 6H 6 the empirical formula is simply CH. 6Molecular formula of benzene C6H6Molecular formula mass of benzene 78Empirical formula of benzene CHEmpirical formula mass of benzene 13.
The ratio of empirical formula is. What is the empirical formula of benzene. What is an empirical formula quizlet.
What is the structure of benzene. Now empirical formula is the simplest ratio of a compounds constituent atoms. The molecular formula of a benzene is C_6H_6.
What is the ratio of empirical formula to molecular formula of benzene. Answer 1 of 2. The molecular formula is the representation of the actual whole number ratio between the elements of the compound.
Answer 1 of 7. The empirical formula of benzene is CH its molecular formula is C 6 H 6. Alkene have a general formula CnH2n therefore the formula of benzene is C4H8.
Empirical formula of a chemical compound is a representation of. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms. Empirical Formula - Learn how to calculate Empirical formula with illustrations.
Mass of empirical formula of benzene CH mass 11213. However the molecular formula is a depiction of the compounds actual whole number ratio. The correct option is A 1.

The Empirical Formula Of Benzene And Acetylene Is Are Youtube

The Various Representations Of Benzene Organic Chemistry Benzene Chemistry Lessons
What Is The Empirical Formula Of Benzene Quora

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
0 Response to "Empirical Formula of Benzene"
Post a Comment